Protein Expression and Purification

Overview of protein expression and purification
What do you know about your protein
How to improve the expression level of active and soluble protein
Strategies for native protein and recombinant protein purification
Methods for protein seperation and protein purification
Custom Protein Service & Contact Us


Overview of Protein Purification and Characterization
AIMS AND OBJECTIVES
 Protein purification has an over 200-year history: the first attempts at isolating substances from plants having similar properties to “egg albumen,” or egg white, were reported in 1789 by Fourcroy. Many proteins from plants were purified in the nineteenth century, though most would not be considered pure by modern standards. A century later, ovalbumin was the first crystalline protein obtained (by Hofmeister in 1889). The year 1989 may not go down in history as a milestone in protein chemistry, but since then there has been a resurgence of interest in proteins after more than a decade of gene excitement.
Protein purification has an over 200-year history: the first attempts at isolating substances from plants having similar properties to “egg albumen,” or egg white, were reported in 1789 by Fourcroy. Many proteins from plants were purified in the nineteenth century, though most would not be considered pure by modern standards. A century later, ovalbumin was the first crystalline protein obtained (by Hofmeister in 1889). The year 1989 may not go down in history as a milestone in protein chemistry, but since then there has been a resurgence of interest in proteins after more than a decade of gene excitement.
The aims of protein purification, up until the 1940s, were simply academic. To then, even the basic facts of protein structure were not fully appreciated, and pure proteins were needed just to study structure and test the rival theories of the pre-DNA days. During the Second World War, an acute need for blood proteins led to development of the Cohn fractionation procedure for purification of albumin and other proteins from serum (Cohn et al., 1946). This was the inception of large-scale protein purifications for commercial purposes; Cohn fractionation continues to be used to this day.
The nature of the proteins studied has also changed substantially. Whereas enzymes were once the most favored subjects, they have now been superceded by nonenzymatic proteins such as growth factors, hormone receptors, viral antigens, and membrane transporters. Many of these occur in minute amounts in the natural source, and their purification can be a major task. Heroic efforts in the past have used kilogram quantities of rather unpleasant starting materials, such as human organs, and ended up with a few micrograms of pure product. It is now more usual, however, to take the genetic approach: clone the gene before the protein has been isolated or even properly identified, and then express it in a suitable host cell culture or organism. The expression level may be orders of magnitude higher than in the original source, which will make purification a relatively simple task. It can be useful to know beforehand some physical properties of the protein, to facilitate the development of a suitable purification protocol from the recombinant source. On the other hand, there are now several ways of preparing fusion proteins, which can be purified by affinity techniques without any knowledge of the properties of the target protein. Moreover, there are ways of modifying the expressed product to simplify purification further.
SOURCES OF MATERIAL FOR PROTEIN PURIFICATION
For many people embarking on a protein purification project, there is no choice of material. They are studying a particular biological tissue or organism, and the objective is to purify a protein from that source. However, there may be approaches that can make the project simpler. If, for instance, the source is difficult to obtain in large amounts, it may be best to carry out at least preliminary trials on a source species more readily obtained. The most obvious and relevant example is when the species being studied is Homo sapiens, and tissue samples are not readily available for practical or ethical reasons, or both. In this case, it is usual to go to where mammalian tissue is readily available (i.e., an abattoir) and work with bovine, ovine, or porcine sources. Alternatively, if quantity of tissue is not a problem, the humble laboratory rat may suffice. Once a protocol for purifying the protein from substitute sources has been worked out, it will be much easier to develop one using human material—the identical procedure may work satisfactorily. Proteins differ to a fairly small extent between species that have diverged within about 100 million years, a time frame that groups together most higher mammals. Thus the behavior of proteins derived from different animals with respect to the various fractionation procedures is likely to be similar, and a protocol worked out for pig tissues is likely to need only minor adjustments for application to human tissues.
METHODS FOR SEPARATION AND PURIFICATION OF PROTEINS
The methods available for protein purification range from simple precipitation procedures used since the nineteenth century to sophisticated chromatographic and affinity techniques that are constantly undergoing development and improvement. Methods can be classified in several alternative ways—perhaps one of the best is based on the properties of the proteins that are being exploited. Thus the methods can be divided into four distinct but interrelated groups depending on protein characteristics: surface features, size and shape, net charge, and bioproperties.
Protein Purification and Characterization Methods Based on Surface Features of Proteins
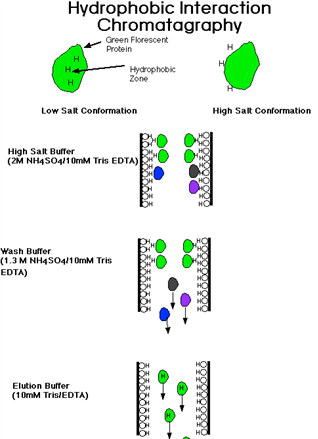 Surface features include charge distribution and accessibility, surface distribution of hydrophobic amino acid side chains, and, to a lesser extent, net charge at a given pH (see discussion of net charge). Methods exploiting surface features mainly depend on solubility properties. Differences in solubility result in precipitation by various manipulations of the solvent in which the proteins are solubilized. Methods for obtaining an extract containing the desired protein in soluble form are given in another chapter. The solvent, nearly always water containing a low concentration of buffer salts, can be treated to alter properties such as ionic strength, dielectric constant, pH, temperature, and detergent content, any of which may selectively precipitate some of the proteins present. Conversely, proteins may be selectively solubilized from an insoluble state by manipulation of the solvent composition. The surface distribution of hydrophobic residues is an important determinant of solubility properties; it is also exploited in hydrophobic chromatography, both in the reversed phase mode and in aqueousphase hydrophobic-interaction chromatography.
Surface features include charge distribution and accessibility, surface distribution of hydrophobic amino acid side chains, and, to a lesser extent, net charge at a given pH (see discussion of net charge). Methods exploiting surface features mainly depend on solubility properties. Differences in solubility result in precipitation by various manipulations of the solvent in which the proteins are solubilized. Methods for obtaining an extract containing the desired protein in soluble form are given in another chapter. The solvent, nearly always water containing a low concentration of buffer salts, can be treated to alter properties such as ionic strength, dielectric constant, pH, temperature, and detergent content, any of which may selectively precipitate some of the proteins present. Conversely, proteins may be selectively solubilized from an insoluble state by manipulation of the solvent composition. The surface distribution of hydrophobic residues is an important determinant of solubility properties; it is also exploited in hydrophobic chromatography, both in the reversed phase mode and in aqueousphase hydrophobic-interaction chromatography.
Although the size and shape of proteins can have some influence on solubility properties, the chief method of exploiting these properties is gel-filtration chromatography. In addition, preparative gel electrophoresis
Protein Purification and Characterization Methods Based on Whole Structure: Size and Shape
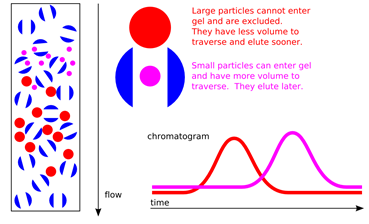 makes use of differences in molecular size. Proteins range in size from the smallest classified as proteins rather than polypeptides, around 5000 Da, up to macromolecular complexes of many million daltons. Many proteins in the bioactive state are oligomers of more than one polypeptide, and these can be dissociated, though normally with loss of overall structure. Thus many proteins have two “sizes”: that of the native state, and that (or those) of the polypeptides in the denatured and dissociated state. Gel-filtration procedures normally deal only with native proteins, whereas electrophoretic procedures commonly involve separation of dissociated and denatured polypeptides.
makes use of differences in molecular size. Proteins range in size from the smallest classified as proteins rather than polypeptides, around 5000 Da, up to macromolecular complexes of many million daltons. Many proteins in the bioactive state are oligomers of more than one polypeptide, and these can be dissociated, though normally with loss of overall structure. Thus many proteins have two “sizes”: that of the native state, and that (or those) of the polypeptides in the denatured and dissociated state. Gel-filtration procedures normally deal only with native proteins, whereas electrophoretic procedures commonly involve separation of dissociated and denatured polypeptides.
Protein Purification and Characterization Methods Based on Net Charge
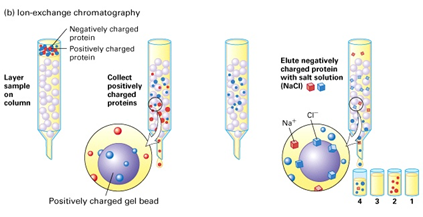 The two techniques that exploit the overall charge of proteins are ion-exchange chromatography (by far the most important) and electrophoresis. Ion exchangers bind charged molecules, and there are essentially only two types of ion exchangers, anion and cation. The net charge of a protein depends on the pH—positive at very low pH, negative at high pH, and zero at some specific point in between, termed the isoelectric point (pI). It should be stressed that at the pI a protein has a great many charges; it just happens that at this pH the total negatives exactly equal the total positives. The most charged state (disregarding the charge sign) is in the pH range 6.0 to 9.0. This is the most stable pH range for most proteins, as it encompasses common physiological pH values. Ion exchangers consist of immobilized charged groups and attract oppositely charged proteins. They provide the mode of separation that has the highest resolution for native proteins. High-performance reversed phase chromatography has equivalent or even better resolution, but it generally involves at least partial denaturation during adsorption and so is not recommended for sensitive proteins such as enzymes. Protein purification using ion-exchange chromatography has mainly employed positively charged anion exchangers, for the simple reason that the majority of proteins at neutral pH are negatively charged (i.e., have a low isoelectric point). Details of methodology are found in following.
The two techniques that exploit the overall charge of proteins are ion-exchange chromatography (by far the most important) and electrophoresis. Ion exchangers bind charged molecules, and there are essentially only two types of ion exchangers, anion and cation. The net charge of a protein depends on the pH—positive at very low pH, negative at high pH, and zero at some specific point in between, termed the isoelectric point (pI). It should be stressed that at the pI a protein has a great many charges; it just happens that at this pH the total negatives exactly equal the total positives. The most charged state (disregarding the charge sign) is in the pH range 6.0 to 9.0. This is the most stable pH range for most proteins, as it encompasses common physiological pH values. Ion exchangers consist of immobilized charged groups and attract oppositely charged proteins. They provide the mode of separation that has the highest resolution for native proteins. High-performance reversed phase chromatography has equivalent or even better resolution, but it generally involves at least partial denaturation during adsorption and so is not recommended for sensitive proteins such as enzymes. Protein purification using ion-exchange chromatography has mainly employed positively charged anion exchangers, for the simple reason that the majority of proteins at neutral pH are negatively charged (i.e., have a low isoelectric point). Details of methodology are found in following.
Protein Purification and Characterization Methods Based on Bioproperties (Affinity)
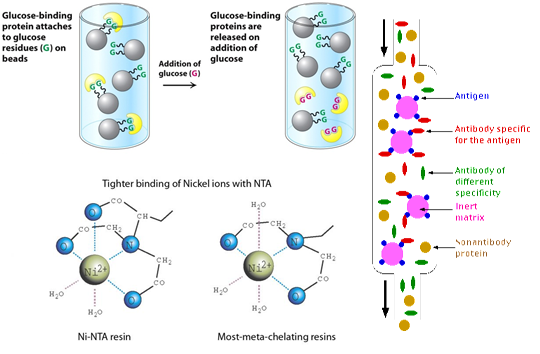 A powerful method for separating the desired protein from others is to use a biospecific method in which the particular biological property of the protein is exploited. The affinity approach is limited to proteins that have a specific binding property, except that proteins are theoretically able to be purified by immunoaffinity chromatography, which is the most specific of all affinity techniques. Most proteins of interest do have a specific ligand: enzymes have substrates and cofactors, and hormone-binding proteins and receptor molecules are designed to bind specifically and tightly to particular hormones and other factors. Immobilization of the ligand to which the protein binds (or of antibody to the protein) enables selective adsorption of the desired protein in the technique known as affinity chromatography. There are also nonchromatographic modes of exploiting biospecific interactions.
A powerful method for separating the desired protein from others is to use a biospecific method in which the particular biological property of the protein is exploited. The affinity approach is limited to proteins that have a specific binding property, except that proteins are theoretically able to be purified by immunoaffinity chromatography, which is the most specific of all affinity techniques. Most proteins of interest do have a specific ligand: enzymes have substrates and cofactors, and hormone-binding proteins and receptor molecules are designed to bind specifically and tightly to particular hormones and other factors. Immobilization of the ligand to which the protein binds (or of antibody to the protein) enables selective adsorption of the desired protein in the technique known as affinity chromatography. There are also nonchromatographic modes of exploiting biospecific interactions.
©2013 BiologicsCorp, All right reserved.
Contact Us


